- Atoms And Elements Bbc
- Atoms And Elements Facts
- Atoms And Elements
- Atoms And Elements Powerpoint
- Atoms And Elements Worksheet Pdf
Identify the building blocks of matter
By contrast, the modern definition of element is couched in terms of a discrete theory of matter, in terms of atoms. The same reference works consulted on the definition of elementall agree when it comes to atom: an atom is the individual structure that constitutes the basic unit of any chemical element. The term atomhas a long history. Introduction to Chemistry, Atoms and Elements Importance of Chemistry Question: If cataclysmic event were to destroy all knowledge of science what would be the most important knowledge to pass on to future generations? Answer: Everything is made of Atoms. Atomic Theory is the central theme of chemistry and most important idea in science.
At its most fundamental level, life is made up of matter. Matter is any substance that occupies space and has mass. Elements are unique forms of matter with specific chemical and physical properties that cannot be broken down into smaller substances by ordinary chemical reactions. There are 118 elements, but only 92 occur naturally. The remaining elements are synthesized in laboratories and are unstable.
Each element is designated by its chemical symbol, which is a single capital letter or, when the first letter is already “taken” by another element, a combination of two letters. Some elements follow the English term for the element, such as C for carbon and Ca for calcium. Other elements’ chemical symbols derive from their Latin names; for example, the symbol for sodium is Na, referring to natrium, the Latin word for sodium.
The four elements common to all living organisms are oxygen (O), carbon (C), hydrogen (H), and nitrogen (N). In the non-living world, elements are found in different proportions, and some elements common to living organisms are relatively rare on the earth as a whole, as shown in Table 1. For example, the atmosphere is rich in nitrogen and oxygen but contains little carbon and hydrogen, while the earth’s crust, although it contains oxygen and a small amount of hydrogen, has little nitrogen and carbon. In spite of their differences in abundance, all elements and the chemical reactions between them obey the same chemical and physical laws regardless of whether they are a part of the living or non-living world.
| Table 1. Approximate Percentage of Elements in Living Organisms (Humans) Compared to the Non-living World | |||
|---|---|---|---|
| Element | Life (Humans) | Atmosphere | Earth’s Crust |
| Oxygen (O) | 65% | 21% | 46% |
| Carbon (C) | 18% | trace | trace |
| Hydrogen (H) | 10% | trace | 0.1% |
| Nitrogen (N) | 3% | 78% | trace |
Learning Objectives
- Draw a diagram of an atom, according to current scientific understanding
- Understand the periodic table of elements and how to use it to understand elements
- Describe the behavior and location of electrons, and how these factors influence bond formation between atoms
Atoms
The Structure of the Atom
To understand how elements come together, we must first discuss the smallest component or building block of an element, the atom. An atom is the smallest unit of matter that retains all of the chemical properties of an element. For example, one gold atom has all of the properties of gold in that it is a solid metal at room temperature. A gold coin is simply a very large number of gold atoms molded into the shape of a coin and containing small amounts of other elements known as impurities. Gold atoms cannot be broken down into anything smaller while still retaining the properties of gold.
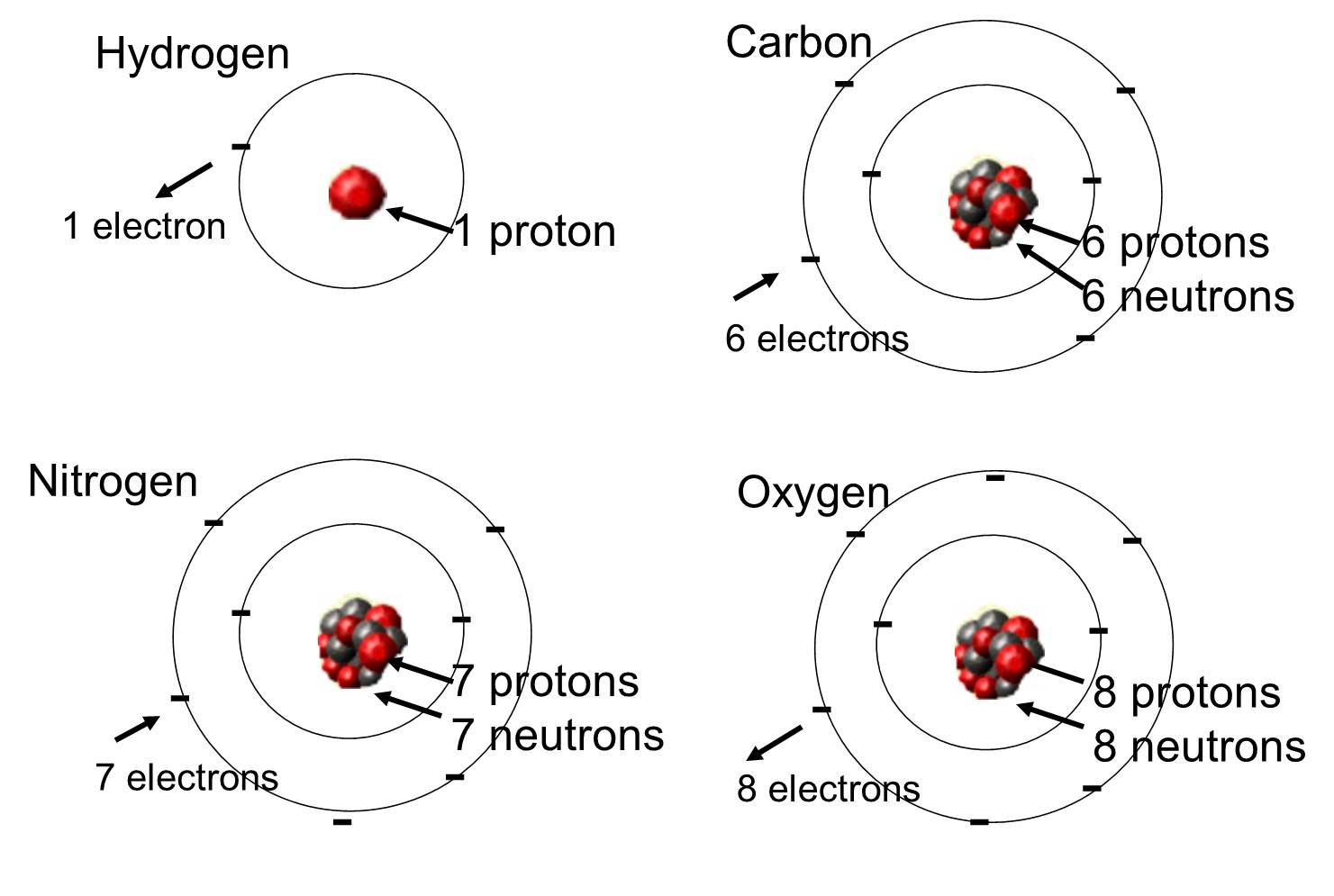
An atom is composed of two regions: the nucleus, which is in the center of the atom and contains protons and neutrons, and the outermost region of the atom which holds its electrons in orbit around the nucleus, as illustrated in Figure 1. Atoms contain protons, electrons, and neutrons, among other subatomic particles. The only exception is hydrogen (H), which is made of one proton and one electron with no neutrons.
Figure 1. Elements, such as helium, depicted here, are made up of atoms. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. Gang beasts crack.
Protons and neutrons have approximately the same mass, about 1.67 × 10–24 grams. Scientists arbitrarily define this amount of mass as one atomic mass unit (amu) or one Dalton, as shown in Table 1. Although similar in mass, protons and neutrons differ in their electric charge. A proton is positively charged whereas a neutron is uncharged. Therefore, the number of neutrons in an atom contributes significantly to its mass, but not to its charge. Electrons are much smaller in mass than protons, weighing only 9.11 × 10–28 grams, or about 1/1800 of an atomic mass unit. Hence, they do not contribute much to an element’s overall atomic mass. Therefore, when considering atomic mass, it is customary to ignore the mass of any electrons and calculate the atom’s mass based on the number of protons and neutrons alone. Although not significant contributors to mass, electrons do contribute greatly to the atom’s charge, as each electron has a negative charge equal to the positive charge of a proton. In uncharged, neutral atoms, the number of electrons orbiting the nucleus is equal to the number of protons inside the nucleus. In these atoms, the positive and negative charges cancel each other out, leading to an atom with no net charge.
Accounting for the sizes of protons, neutrons, and electrons, most of the volume of an atom—greater than 99 percent—is, in fact, empty space. With all this empty space, one might ask why so-called solid objects do not just pass through one another. The reason they do not is that the electrons that surround all atoms are negatively charged and negative charges repel each other.
| Table 1. Protons, Neutrons, and Electrons | |||
|---|---|---|---|
| Charge | Mass (amu) | Location | |
| Proton | +1 | 1 | nucleus |
| Neutron | 0 | 1 | nucleus |
| Electron | –1 | 0 | orbitals |
Atomic Number and Mass
Atoms of each element contain a characteristic number of protons and electrons. The number of protons determines an element’s atomic number and is used to distinguish one element from another. The number of neutrons is variable, resulting in isotopes, which are different forms of the same atom that vary only in the number of neutrons they possess. Together, the number of protons and the number of neutrons determine an element’s mass number, as illustrated in Figure 2. Note that the small contribution of mass from electrons is disregarded in calculating the mass number. This approximation of mass can be used to easily calculate how many neutrons an element has by simply subtracting the number of protons from the mass number. Since an element’s isotopes will have slightly different mass numbers, scientists also determine the atomic mass, which is the calculated mean of the mass number for its naturally occurring isotopes. Often, the resulting number contains a fraction. For example, the atomic mass of chlorine (Cl) is 35.45 because chlorine is composed of several isotopes, some (the majority) with atomic mass 35 (17 protons and 18 neutrons) and some with atomic mass 37 (17 protons and 20 neutrons).
Practice Question
Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively. Its atomic mass is 12.11.

How many neutrons do carbon-12 and carbon-13 have, respectively?
Show AnswerThe Periodic Table of Elements
The different elements are organized and displayed in the periodic table. Devised by Russian chemist Dmitri Mendeleev (1834–1907) in 1869, the table groups elements that, although unique, share certain chemical properties with other elements. The properties of elements are responsible for their physical state at room temperature: they may be gases, solids, or liquids. Elements also have specific chemical reactivity, the ability to combine and to chemically bond with each other.
In the periodic table, shown in Figure 3, the elements are organized and displayed according to their atomic number and are arranged in a series of rows and columns based on shared chemical and physical properties. In addition to providing the atomic number for each element, the periodic table also displays the element’s atomic mass. Looking at carbon, for example, its symbol (C) and name appear, as well as its atomic number of six (in the upper left-hand corner) and its atomic mass of 12.11.
Figure 3. The periodic table shows the atomic mass and atomic number of each element. The atomic number appears above the symbol for the element and the approximate atomic mass appears below it.
The periodic table groups elements according to chemical properties. The differences in chemical reactivity between the elements are based on the number and spatial distribution of an atom’s electrons. Atoms that chemically react and bond to each other form molecules. Molecules are simply two or more atoms chemically bonded together. Logically, when two atoms chemically bond to form a molecule, their electrons, which form the outermost region of each atom, come together first as the atoms form a chemical bond.
Watch this video for a more in-depth introduction to the periodic table:
Electrons
Electron Shells and the Bohr Model
It should be stressed that there is a connection between the number of protons in an element, the atomic number that distinguishes one element from another, and the number of electrons it has. In all electrically neutral atoms, the number of electrons is the same as the number of protons. Thus, each element, at least when electrically neutral, has a characteristic number of electrons equal to its atomic number.
An early model of the atom was developed in 1913 by Danish scientist Niels Bohr (1885–1962). In this model, electrons exist within principal shells. An electron normally exists in the lowest energy shell available, which is the one closest to the nucleus. Energy from a photon of light can bump it up to a higher energy shell, but this situation is unstable, and the electron quickly decays back to the ground state. In the process, a photon of light is released.
The Bohr model shows the atom as a central nucleus containing protons and neutrons, with the electrons in circular orbitals at specific distances from the nucleus, as illustrated in Figure 4. These orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the outermost shells. These energy levels are designated by a number and the symbol “n.” For example, 1n represents the first energy level located closest to the nucleus.
Electrons fill orbitals in a consistent order: they first fill the orbitals closest to the nucleus, then they continue to fill orbitals of increasing energy further from the nucleus. If there are multiple orbitals of equal energy, they will be filled with one electron in each energy level before a second electron is added. The electrons of the outermost energy level determine the energetic stability of the atom and its tendency to form chemical bonds with other atoms to form molecules.
Under standard conditions, atoms fill the inner shells first, often resulting in a variable number of electrons in the outermost shell. The innermost shell has a maximum of two electrons but the next two electron shells can each have a maximum of eight electrons. This is known as the octet rule, which states, with the exception of the innermost shell, that atoms are more stable energetically when they have eight electrons in their valence shell, the outermost electron shell. Examples of some neutral atoms and their electron configurations are shown in Figure 5. Notice that in this figure, helium has a complete outer electron shell, with two electrons filling its first and only shell. Similarly, neon has a complete outer 2n shell containing eight electrons. In contrast, chlorine and sodium have seven and one in their outer shells, respectively, but theoretically they would be more energetically stable if they followed the octet rule and had eight.
Practice Question
Figure 5. Bohr diagrams for hydrogen, helium, lithium, carbon, fluorine, neon, sodium, silicon, chlorine, and argon.
Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown in Figure 5) have a full outer, or valence, shell. A full valence shell is the most stable electron configuration. Elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable electron configuration.
An atom may give, take, or share electrons with another atom to achieve a full valence shell, the most stable electron configuration. Looking at this figure, how many electrons do elements in group 1 need to lose in order to achieve a stable electron configuration? How many electrons do elements in groups 14 and 17 need to gain to achieve a stable configuration?
Show AnswerUnderstanding that the organization of the periodic table is based on the total number of protons (and electrons) helps us know how electrons are distributed among the outer shell. The periodic table is arranged in columns and rows based on the number of electrons and where these electrons are located. Take a closer look at the some of the elements in the periodic table’s far right column in Figure 3.
The group 18 atoms helium (He), neon (Ne), and argon (Ar) all have filled outer electron shells, making it unnecessary for them to share electrons with other atoms to attain stability; they are highly stable as single atoms. Their non-reactivity has resulted in their being named the inert gases (or noble gases). Compare this to the group 1 elements in the left-hand column. These elements, including hydrogen (H), lithium (Li), and sodium (Na), all have one electron in their outermost shells. That means that they can achieve a stable configuration and a filled outer shell by donating or sharing one electron with another atom or a molecule such as water. Hydrogen will donate or share its electron to achieve this configuration, while lithium and sodium will donate their electron to become stable. As a result of losing a negatively charged electron, they become positively charged ions. Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules, making them negatively charged ions. Group 14 elements, of which carbon is the most important to living systems, have four electrons in their outer shell allowing them to make several covalent bonds (discussed below) with other atoms. Thus, the columns of the periodic table represent the potential shared state of these elements’ outer electron shells that is responsible for their similar chemical characteristics.
Electron Orbitals
Although useful to explain the reactivity and chemical bonding of certain elements, the Bohr model of the atom does not accurately reflect how electrons are spatially distributed surrounding the nucleus. They do not circle the nucleus like the earth orbits the sun, but are found in electron orbitals. These relatively complex shapes result from the fact that electrons behave not just like particles, but also like waves. Mathematical equations from quantum mechanics known as wave functions can predict within a certain level of probability where an electron might be at any given time. The area where an electron is most likely to be found is called its orbital.
Figure 6. Click for a larger image. The s subshells are shaped like spheres. Both the 1n and 2n principal shells have an s orbital, but the size of the sphere is larger in the 2n orbital. Leviathan: the cargo — ongoing series crack. Each sphere is a single orbital. p subshells are made up of three dumbbell-shaped orbitals. Principal shell 2n has a p subshell, but shell 1 does not.
Recall that the Bohr model depicts an atom’s electron shell configuration. Within each electron shell are subshells, and each subshell has a specified number of orbitals containing electrons. While it is impossible to calculate exactly where an electron is located, scientists know that it is most probably located within its orbital path. Subshells are designated by the letters s, p, d, and f. The s subshell is spherical in shape and has one orbital. Principal shell 1n has only a single s orbital, which can hold two electrons. Principal shell 2n has one s and one p subshell, and can hold a total of eight electrons. The p subshell has three dumbbell-shaped orbitals, as illustrated in Figure 6. Subshells d and f have more complex shapes and contain five and seven orbitals, respectively. These are not shown in the illustration. Principal shell 3n has s, p, and d subshells and can hold 18 electrons. Principal shell 4n has s, p, d and f orbitals and can hold 32 electrons. Moving away from the nucleus, the number of electrons and orbitals found in the energy levels increases. Progressing from one atom to the next in the periodic table, the electron structure can be worked out by fitting an extra electron into the next available orbital.
The closest orbital to the nucleus, called the 1s orbital, can hold up to two electrons. This orbital is equivalent to the innermost electron shell of the Bohr model of the atom. It is called the 1s orbital because it is spherical around the nucleus. The 1s orbital is the closest orbital to the nucleus, and it is always filled first, before any other orbital can be filled. Hydrogen has one electron; therefore, it has only one spot within the 1s orbital occupied. This is designated as 1s1, where the superscripted 1 refers to the one electron within the 1s orbital. Helium has two electrons; therefore, it can completely fill the 1s orbital with its two electrons. This is designated as 1s2, referring to the two electrons of helium in the 1s orbital. On the periodic table Figure 6, hydrogen and helium are the only two elements in the first row (period); this is because they only have electrons in their first shell, the 1s orbital. Hydrogen and helium are the only two elements that have the 1s and no other electron orbitals in the electrically neutral state.
The second electron shell may contain eight electrons. This shell contains another spherical s orbital and three “dumbbell” shaped p orbitals, each of which can hold two electrons, as shown in Figure 6. After the 1s orbital is filled, the second electron shell is filled, first filling its 2s orbital and then its three p orbitals. When filling the p orbitals, each takes a single electron; once each p orbital has an electron, a second may be added. Lithium (Li) contains three electrons that occupy the first and second shells. Two electrons fill the 1s orbital, and the third electron then fills the 2s orbital. Its electron configuration is 1s22s1. Neon (Ne), on the other hand, has a total of ten electrons: two are in its innermost 1s orbital and eight fill its second shell (two each in the 2s and three p orbitals); thus, it is an inert gas and energetically stable as a single atom that will rarely form a chemical bond with other atoms. Larger elements have additional orbitals, making up the third electron shell. While the concepts of electron shells and orbitals are closely related, orbitals provide a more accurate depiction of the electron configuration of an atom because the orbital model specifies the different shapes and special orientations of all the places that electrons may occupy.
Watch this visual animation to see the spatial arrangement of the p and s orbitals. Note that this video has no audio.
Video Review
This video gives another overview of the electron:
Check Your Understanding
Answer the question(s) below to see how well you understand the topics covered in the previous section. This short quiz does not count toward your grade in the class, and you can retake it an unlimited number of times.
Use this quiz to check your understanding and decide whether to (1) study the previous section further or (2) move on to the next section.
| Index to this page |
Elements
Atoms And Elements Bbc
Elements consist of only one kind of atom and cannot be decomposed into simpler substances.
Our planet is made up of some 90 elements. (Tiny amounts — sometimes only a few atoms — of additional elements have been made in nuclear physics laboratories, but they play no role in our story). Of these 90, only 25 or so are used to build living things.
The table shows the 11 most prevalent elements in the lithosphere (the earth's crust) and in the human body.
| Elemental composition of the lithosphere and the human body. Each number represents the percent of the total number of atoms present. For example, 47 of every 100 atoms found in a representative sample of the lithosphere are oxygen while there are only 19 atoms of carbon in every 10,000 atoms of lithosphere. | |||
| Composition of the Lithosphere | Composition of the Human Body | ||
| Oxygen | 47 | Hydrogen | 63 |
| Silicon | 28 | Oxygen | 25.5 |
| Aluminum | 7.9 | Carbon | 9.5 |
| Iron | 4.5 | Nitrogen | 1.4 |
| Calcium | 3.5 | Calcium | 0.31 |
| Sodium | 2.5 | Phosphorus | 0.22 |
| Potassium | 2.5 | Chlorine | 0.03 |
| Magnesium | 2.2 | Potassium | 0.06 |
| Titanium | 0.46 | Sulfur | 0.05 |
| Hydrogen | 0.22 | Sodium | 0.03 |
| Carbon | 0.19 | Magnesium | 0.01 |
| All others | <0.1 | All others | <0.01 |
- uses only a fraction of the elements available to it
- but, as the table shows, the relative proportions of those it does acquire from its surroundings are quite different from the proportions in the environment.
- the composition of living things is not simply a reflection of the elements available to them
For example, hydrogen, carbon, and nitrogen together represent less than 1% of the atoms found in the earth's crust but some 74% of the atoms in living matter.
- one of the properties of life is to take up certain elements that are scarce in the nonliving world and concentrate them within living cells.
Some sea animals accumulate elements like vanadium and iodine within their cells to concentrations a thousand or more times as great as in the surrounding sea water. It has even been proposed that uranium be 'mined' from the sea by extracting it from certain algae that can take up uranium from sea water and concentrate it within their cells.
There is still some uncertainty about the exact number of elements required by living things. Some elements, e.g., aluminum, are found in tiny amounts in living tissue, but whether they are playing an essential role or are simply an accidental acquisition (aluminum probably is) is sometimes difficult to determine.
Atoms
Each element is made up of one kind of atom. We can define an atom as the smallest part of an element that can enter into combination with other elements.Structure of the atom
Each atom consists of- a small, dense, positively-charged nucleus surrounded by
- much lighter, negatively-charged electrons.
- a single positively-charged proton. Because of its single proton, the atom of hydrogen is assigned an atomic number of 1.
- a single electron.
The charge of the electron is the same magnitude as that of the proton, so the atom as a whole is electrically neutral. Its proton accounts for almost all the weight of the atom.
The nucleus of the atom of the element helium (He) has- two protons (hence helium has an atomic number of 2) and
- two neutrons. Neutrons have the same weight as protons but no electrical charge.
Atoms And Elements Facts
The helium atom has two electrons so that, once again, the atom as a whole is neutral.
Atoms And Elements
The structure of each of the other kinds of atoms follows the same plan. From Lithium (At. No. = 3) to uranium (At. No. = 92), the atoms of each element can be listed in order of increasing atomic number. There are no gaps in the list. Each element has a unique atomic number and its atoms have one more proton and one more electron than the atoms of the element that precedes it in the list.
Electrons
| Atomic Number | Element | Energy Levels or 'shells' | ||||
| K | L | M | N | O | ||
| 1 | Hydrogen (H) | 1 | ||||
| 2 | Helium (He) | 2 | ||||
| 3 | Lithium (Li) | 2 | 1 | |||
| 4 | Beryllium (Be) | 2 | 2 | |||
| 5 | Boron (B) | 2 | 3 | |||
| 6 | Carbon (C) | 2 | 4 | |||
| 7 | Nitrogen (N) | 2 | 5 | |||
| 8 | Oxygen (O) | 2 | 6 | |||
| 9 | Fluorine (F) | 2 | 7 | |||
| 10 | Neon (Ne) | 2 | 8 | |||
| 11 | Sodium (Na) | 2 | 8 | 1 | ||
| 12 | Magnesium (Mg) | 2 | 8 | 2 | ||
| 13 | Aluminum (Al) | 2 | 8 | 3 | ||
| 14 | Silicon (Si) | 2 | 8 | 4 | ||
| 15 | Phosphorus (P) | 2 | 8 | 5 | ||
| 16 | Sulfur (S) | 2 | 8 | 6 | ||
| 17 | Chlorine (Cl) | 2 | 8 | 7 | ||
| 18 | Argon (Ar) | 2 | 8 | 8 | ||
| 19 | Potassium (K) | 2 | 8 | 8 | 1 | |
| 20 | Calcium (Ca) | 2 | 8 | 8 | 2 | |
| 21 | Scandium (Sc) | 2 | 8 | 9 | 2 | |
| 22 | Titanium (Ti) | 2 | 8 | 10 | 2 | |
| 23 | Vanadium (V) | 2 | 8 | 11 | 2 | |
| 24 | Chromium (Cr) | 2 | 8 | 13 | 1 | |
| 25 | Manganese (Mn) | 2 | 8 | 13 | 2 | |
| 26 | Iron (Fe) | 2 | 8 | 14 | 2 | |
| 27 | Cobalt (Co) | 2 | 8 | 15 | 2 | |
| 28 | Nickel (Ni) | 2 | 8 | 16 | 2 | |
| 29 | Copper (Cu) | 2 | 8 | 18 | 1 | |
| 30 | Zinc (Zn) | 2 | 8 | 18 | 2 | |
| 31 | Gallium (Ga) | 2 | 8 | 18 | 3 | |
| 32 | Germanium (Ge) | 2 | 8 | 18 | 4 | |
| 33 | Arsenic (As) | 2 | 8 | 18 | 5 | |
| 34 | Selenium (Se) | 2 | 8 | 18 | 6 | |
| 35 | Bromine (Br) | 2 | 8 | 18 | 7 | |
| 36 | Krypton (Kr) | 2 | 8 | 18 | 8 | |
| 42 | Molybdenum (Mo) | 2 | 8 | 18 | 13 | 1 |
| 48 | Cadmium (Cd) | 2 | 8 | 18 | 18 | 2 |
| 50 | Tin (Sn) | 2 | 8 | 18 | 18 | 4 |
| 53 | Iodine (I) | 2 | 8 | 18 | 18 | 7 |
Electrons are confined to relatively discrete regions around the nucleus. The two electrons of helium, for example, are confined to a spherical zone surrounding the nucleus called the K shell or K energy level.
Lithium (At. No. = 3) has three electrons, two in the K shell and one located farther from the nucleus in the L shell. Being farther away from the opposite (+) charges of the nucleus, this third electron is held less tightly.
Each of the following elements, in order of increasing atomic number, adds one more electron to the L shell until we reach neon (At. No. = 10) which has eight electrons in the L shell.
Sodium places its eleventh electron in a still higher energy level, the M shell.
From sodium to argon, this shell is gradually filled with electrons until, once again, a maximum of eight is reached.
Note that after the K shell with its maximum of two electrons, the maximum number of electrons in any other outermost shell is eight.
As we shall see, the chemical properties of each element are strongly influenced by the number of electrons in its outermost energy level (shell).
This table shows the electronic structure of the atoms of elements 1 – 36 with those that have been demonstrated to be used by living things shown in red. Four elements of still higher atomic numbers that have been shown to be used by living things are also included.
The electronic structure of an atom plays the major role in its chemistry.
The pattern of electrons in an atom — especially those in the outermost shell — determines- the valence of the atom; that is, the ratios with which it interacts with other atoms, and to a large degree,
- the electronegativity of the atom; that is, the strength with which it attracts other electrons.
| Link to a discussion of electronegativity. |
Example 1: Fluorine, chlorine, bromine, and iodine each have 7 electrons in their outermost shell. These so-called halogens are also quite similar in their chemical behavior. When dissolved in water, for example, they all produce germicidal solutions.
Example 2: Those elements with 1, 2, or 3 electrons in their outermost shell are the metals.
Example 3: Those elements with 4, 5, 6, or 7 in their outermost shell are the nonmetals.
Example 4: Helium (with its 2), neon, argon, and krypton (each with 8) have 'filled' their outermost shells. They are the so-called inert or 'noble' gases. They have no chemistry at all. Under normal conditions they do not interact with other atoms.So, it is the number and arrangement of the electrons in the atoms of an element that establish the chemical behavior of that element.
This is how it works.
The atoms of an element interact with other atoms in such ways and ratios that they can 'fill' their outermost shell with 8 electrons (2 for hydrogen). They may do this by- acquiring more electrons from another atom
- losing electrons to another atom
- sharing electrons with another atom
Atoms And Elements Powerpoint
The number of electrons that an atom must acquire, or lose, or share to reach a stable configuration of 8 (2 for hydrogen) is called its valence.
Hydrogen, lithium, sodium, and potassium atoms all have a single electron in their outermost shell. Fluorine, chlorine, bromine, and iodine atoms all have 7. Any atom of the first group will interact with a single atom of any of the second group forming, HCl, NaCl, KI, etc. The result of all of these interactions is a pair of atoms each with an outermost shell like that of one of the inert gases: 2 for hydrogen, 8 for the others.
The elements with 2 electrons in their outermost shell interact with chlorine and the other halogens to form, e.g., BeCl2, MgCl2, CaCl2. Again, the result is a pair of atoms each with a stable octet of electrons in its outermost shell.
The elements with 3 electrons in their outermost shell will interact with chlorine in a ratio of 1:3, forming BCl3, AlCl3.
Carbon atoms, with their 4 electrons in the L shell interact with chlorine to form CCl4.
Nitrogen, with its 5 outermost electrons, interacts with hydrogen atoms in a ratio of 1:3, forming ammonia (NH3).
Oxygen and sulfur, with their 6 outermost electrons react with hydrogen to form water (H2O) and hydrogen sulfide (H2S).
What determines whether a pair of atoms swap or share electrons?
The answer is their relative electronegativities. If two atoms differ greatly in their affinity for electrons; that is, in their electronegativity, then the strongly electronegative atom will take the electron away from the weakly electronegative one.
Example: Na (weakly electronegative) gives up its single electron to an atom of chlorine (strongly electronegative) to form NaCl. The sodium atom now has only 10 electrons but still 11 protons so there is a net positive charge of one on the atom. Similarly, chlorine now has one more electron than proton so its now has a net negative charge of 1. Electrically charged atoms are called ions. The mutual attraction of opposite electrical charges holds the ions together by ionic bonds.
Example: Carbon and hydrogen are both only weakly electronegative so neither can remove electrons from the other. Instead they achieve a stable configuration by sharing their outermost electrons forming covalent bonds of CH4.
| Link to an expanded discussion of how electronegativity influences the types of bonds that form between atoms. |
Isotopes
The number of protons in the nucleus of its atoms, which is its atomic number, defines each element. However, the nuclei of a given element may have varying numbers of neutrons. Because neutrons have weight (about the same as that of protons), such atoms differ in the atomic weight.
Atoms of the same element that differ in their atomic weight are called isotopes.
Atomic weights are expressed in terms of a standard atom: the isotope of carbon that has 6 protons and 6 neutrons in its nucleus. This atom is designated carbon-12 or 12C. It is arbitrarily assigned an atomic weight of 12 daltons (named after John Dalton, the pioneer in the study of atomic weights). Thus a dalton is 1/12 the weight of an atom of 12C. Both protons and neutrons have weights very close to 1 dalton each. Carbon-12 is the most common isotope of carbon. Carbon-13 (13C) with 6 protons and 7 neutrons, and carbon-14 (14C) with 6 protons and 8 neutrons are found in much smaller quantities.
Isotopes as 'tracers'
Atoms And Elements Worksheet Pdf
One can prepare, for example, a carbon compound used by living things that has many of its normal 12C atoms replaced by 14C atoms. Carbon-14 happens to be radioactive. By tracing the fate of radioactivity within the organism, one can learn the normal pathway of this carbon compound in that organism. Thus 14C serves as an isotopic 'label' or 'tracer'.
The basis of this technique is that the weight of the nucleus of an atom has little or no effect on the chemical properties of that atom. The chemistry of an element and the atoms of which it is made — whatever their atomic weight — is a function of the atomic number of that element. As long as the atom had 6 protons, it is an atom of carbon irrespective of the number of neutrons. Thus while 6 protons and 8 neutrons produce an isotope of carbon, 14C, 7 protons and 7 neutrons produce a totally-different element, nitrogen-14.
| Welcome&Next Search |