Ozone (O 3) is formed by electrostatic discharge in the presence of molecular oxygen. A double oxygen molecule (O 2) 2 is known, found as a minor component of liquid oxygen. Epoxides are ethers in which the oxygen atom is part of a ring of three atoms. Oxygen is in group 16/VIA, so it has six valence electrons. Draw the symbol for oxygen. Then place one dot at each side of the symbol. There are now four unpaired electrons around the oxygen symbol. There are also two more valence electrons and they are paired with two of the unpaired electrons. The Atom is a legacy super-hero name, primarily associated with the ability to shrink in size. The original was Al Pratt during the Golden Age, a diminutive man with superhuman strength; he was a founding member of the Justice Society. Ray Palmer took the name during the Silver Age, a physics professor at Ivy University who developed equipment to shrink himself down to subatomic levels while. Atom is an award-winning app and website that makes it easy to find new movie releases playing in theaters near you. Buy tickets for the latest movie showtimes and hot movies out this week plus special movie events in theaters.
What is the electron dot diagram for an oxygen atom?
2 Answers
Explanation:
Now, this is only one way we can draw the electron dot diagram for Oxygen. So as you may of remember from Chemistry class, before it can pair up on any other sides, it needs to have 1 electron per side of the letter then it can double up. Oxygen has a special rule when doubling. It must be adjacent like shown below.

Types Of Atoms
Explanation:
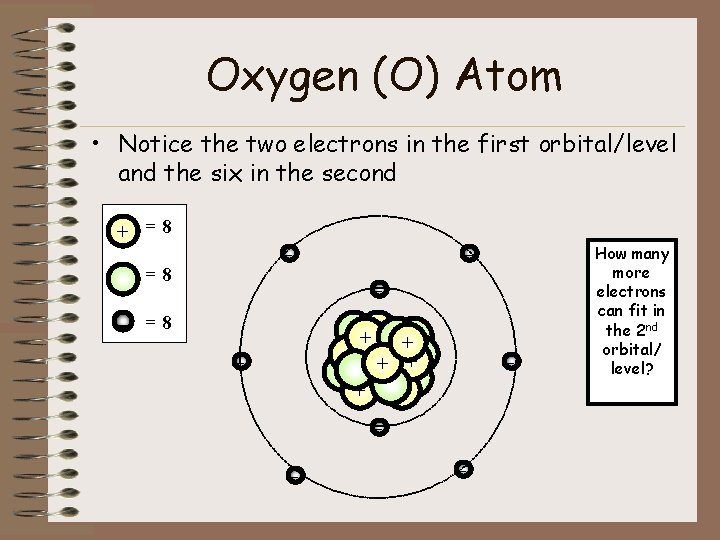
The electron dot diagram for an element shows the valence electrons for the element. Oxygen is in group 16/VIA, so it has six valence electrons.
Draw the symbol for oxygen. Then place one dot at each side of the symbol. There are now four unpaired electrons around the oxygen symbol. There are also two more valence electrons and they are paired with two of the unpaired electrons. This will give you one pair of electrons on two sides of the symbol, and the other two sides will each have an unpaired electron.
O Atomic Mass
Related questions
