A force-plate actometer consists of a rigid, low mass floor supported by force sensors whose force-proportional electronic signals are processed by a computer to allow the computation of a subject’s x – y location in the horizontal plane, and, at the same time, to record the time series of reactive forces as the subject moves on the plate. The ActTrust Actigraph measures special parameters at the wrist to examine sleep and chronobiology. Designed specifically for medical and research applications, the device is a reliable and intuitive tool for scientists, physicians and therapists. Founded by a visionary professional Orchid scientific & Innovative India Pvt. Ltd., is a vision translated into reality. Founders' experience, expertise and vision in the field of pharmaceutical equipment rendered Orchid Scientific & Innovative India Pvt. Ltd., to be the youngest face in manufacturing and supplying of pharmaceutical research, preclinical research and laboratory animal housing. Actigraphy technology can help you assess the sleep/wake patterns of a variety of patients with various sleep complaints. Actigraphy serves as a complement or adjunct to a polysomnogram or subjective paper diary.
A new wrist-worn gadget may help people with sleep problems by objectively tracking their real-life sleep habits and quality, scientists say. The gadget, called actimeter, records data on wrist movement from which one can obtain activity patterns for up to three months.
The researchers from the Ludwig Maximilian University of Munich (LMU) in Germany used the actimeters to assess
rest/activity cycles not just over the course of the waking day, but also during sleep itself. The findings are the latest in a larger, ongoing human sleep project, designed to learn more about sleep and its essential role in our lives by collecting sleep data on thousands of people in the real world.
“This will help many who have sleep problems and will hopefully increase the appreciation for the importance of
sleep for our health and well-being,” Till Roenneberg from LMU Munich said. The team of researchers had been collecting information on sleep duration and quality via questionnaire. The next step was to find a way to collect objective measurements of sleep characteristics on similarly large numbers of people.
In the new study published in the journal Current Biology, the researchers looked at actimeter data collected over more than 20,000 days from 574 subjects, aged 8 to 92 years. However, the patterns of activity during sleep collected using the devices appeared rather messy. It was hard to discern the cyclical sleep patterns normally seen with other, more complicated devices in the lab. By focusing on periods of inactivity during the night, a much clearer cyclical pattern began to emerge.
They used a simple conversion to measure inactivity (as opposed to activity) on a scale of near zero to 100, with 100 representing total inactivity. “It was flabbergasting how it clarified the structures,” Roenneberg said. The researchers called the new measure “locomotor inactivity during sleep” (LIDS). Those measures showed that movement patterns reflect sleep cycles and replicate the dynamics seen in the lab.
“Many devices have tried to use activity to assess sleep structures, but our method is simple, transparent, and works especially in long-term recordings,” Roenneberg said.
COMPARATIVE ANALYSIS OF DRUG-INDUCED PARKINSONISM LIKE BEHAVIORS IN C57BL6 BLACK MICE USING A FORCE PLATE ACTIMETER.
HTML Full TextCOMPARATIVE ANALYSIS OF DRUG-INDUCED PARKINSONISM LIKE BEHAVIORS IN C57BL6 BLACK MICE USING A FORCE PLATE ACTIMETER.
Stuti Shrestha 1, Srichan Phornchirasilp 1 and Krittiya Thisayakorn *2
Department of Pharmacology 1, Faculty of Pharmacy, Mahidol University. Thailand.
Pharmaceutical and Natural product Department 2, Thailand Institute of Scientific and Technological Research (TISTR), PathumThani, Thailand.
ABSTRACT: This study aims to compare the Parkinsonism behavior induced by two drugs: Tacrine (9-amino-1,2,3,4-tetrahydroaminoacridine hydrochloride hydrate and MPTP (1-methyl, 4-phenyl 1,2,3,6 tetrahydropyrolidine) using a Force Plate Actimeter (FPA). 25 C57BL/6 black mice were equally divided into two groups for 5mg/kg tacrine and 30mg/kg MPTP experiment. Both groups contained sham, 5mg/kg tacrine or 30mg/kg MPTP group and sinemet group (oral administration of 10mg/kg sinemet (levodopa:carbidopa in 4:1) prior to intraperitoneal injection of 5mg/kg tacrine or 30mg/kg MPTP). Mice were immediately kept inside FPA to record their behavior. Distance travelled, bout of low mobility (BLM) and power spectra of parkinsonian symptoms shown by both drugs were computed. Both neurotoxins reduced the motor abilities of mice in comparison with sham. Oral treatment of sinemet improved their disability. According to power spectra analysis, tacrine had only one significant narrow peak from 10-12 Hz while MPTP exhibited one to four peaks from 8 to 12 Hz. This might be because the behavior expected from tacrine is lateral movement of jaw (facial part) whilst that of MPTP is rigidity and tremor (facial, trunk and tail portion). This experiment indicates that both tacrine and MPTP can possibly impair motor functions of C57BL/6 black mice. It is possible to use these results to create Parkinsonism model in laboratory by the use of FPA. This model may be used to evaluate capability of new anti parkinsonian drugs to improve motor abilities in rodents.
| Keywords: |
Parkinson’s disease,
Motor functions, Tacrine,

MPTP, FPA..
INTRODUCTION: Parkinson’s disease (PD) is a common neurodegenerative disorder that mainly affects elderly people above the age of 60 1, 2. Motor symptoms of PD like tremor, stiffness, slow gait and postural instability are directly linked to progressive loss of dopamine neurons of nigrostriatal pathway 3, 4. Nigrostriatal pathway is one of the dopaminergic pathways that connect substantia nigra with striatum.
Substantia nigra pars compacta (SNpc) is a structure of the brain that is found in midbrain (mesencephalon) and has a major role in controlling movement of body by supplying sufficient dopamine to striatum via nigrostriatal pathway. This whole system is responsible for smooth muscle coordination and movement of body under normal physiological condition 5, 6.
In case of PD, there is severe loss of dopaminergic neurons in SNpc. The level of dopamine is drastically reduced in striatum, leading to characteristic impairment of movement and locomotion 5, 7. Apart from their cardinal motor features, patient with PD also display hypokinesia and rigidity of or facial muscles. Due to this, patients show symptoms like difficulty in swallowing, reduced eyes blinking and involuntary jaw movement 8, 9.
Tacrine (9-amino-1,2,3,4-tetra hydro amino acridine hydrochloride hydrate) belongs to parasympathomimetics and acetyl-cholinesterase inhibitor group. Tacrine acts by reversibly binding and inactivating cholinesterases 10, 11. Thus, it inhibits the hydrolysis of acetylcholine from cholinergic neurons and increases endogenous levels of neurotransmitter acetylcholine at cholinergic synapses 10, 12. Previous studies have shown that tacrine can stimulate muscarinic cholinergic receptors and triggers different movement of facial muscles like rapid and vertical deflection of lower jaw 10, 11. These deflections are purposelessly and are not encouraged by any motivation 12. TJMs are induced by conditions that lead to parkinsonism in humans and have been validated as a valuable model of parkinsonian tremor 10, 13.
MPTP (1-methyl, 4-phenyl 1,2,3,6 tetrahydro pyrolidine) is a lipid soluble neurotoxin that shows its lethal action by the inhibiting complex I of Electron Transport Chain (ETC) in mitochondria 14. It is selectively toxic to dopaminergic neurons of the nigrostriatal pathway. Inhibition of Complex I can also trigger the formation of reactive oxygen species that initiates DNA strands breakage leading to neuronal cell death. In laboratory, MPTP is used to produce parkinsonism like behavior such as tremor, rigidity, akinesia 15, 16.
Wang, et al in 2001 showed that harmaline and amphetamine induce narrow band increase in 10-12Hz region of power spectra by the use of Force Plate Actimeter (FPA) 17. Now, by manipulating this experiment, the value of frequency required for MPTP and tacrine to show their effects is studied. Many studies have been conducted in the field of MPTP and tacrine in their induction of Parkinsonism syndrome in laboratory.
However, the behavioral comparison between these two drugs has not been done before. Therefore, this article aims to study the behavioral comparison of MPTP and tacrine in C57BL/6 black mice using FPA.
Device:
FPA is especially designed with an objective of behavior quantification of rodents and other small laboratory animals. It can extract much functional information from the behaviors shown by rodents after the drug is administered, for example, locomotor activity, tremor, distance travelling, gait disturbances, and rhythmicity. Hence, it has been very useful for studying the neurological effects of drugs in the animals. A rigid, horizontal graphite plate is supported by four force transducers. The animal is placed on the graphite plate and left freely. When it moves on the plate, the movement is sensed by transducers whose signal is further processed by computer to give data of behavior shown by the animal during the recording 18.
It can record both force of whole body tremor and locomotion of same animal at same time. This instrument is used to quantify the behavioral attributes shown by toxin after its administration, which is required for neuroscience 18. Therefore, we can describe quantitatively the frequency characteristics of jaw movement and tremor induced by two drugs (tacrine and MPTP) respectively and also locomotor activity effects along with other abnormal behaviors on rodents using a force-plate actimeter.
METHODS:
Animals:
A total of 25 adult C57BL/6 black mice (25-35 g, from National Laboratory Center, Salaya, Nakorn Pathom) were used as subject in the study. Animals were housed in cages under standard and controlled conditions of humidity (50-70%), lighting (12 h light/dark cycle) and temperature (22±2 ºC), with ad libitum water and diet. The protocol of animal handling and procedure was approved by the Animal Care and Use Committee, Thailand Institute of Scientific Technological Research (TISTR) and Animal Ethic Committee of Faculty of Pharmacy, Mahidol University.
Treatment:
Animals were randomly divided into two groups, 5mg/kg tacrine group and 30mg/kg MPTP group. Tacrine group was further divided into:
Sham: Oral administration of 3% (w/v) acacia followed by intraperitoneal (i.p.) injection of Normal Saline Solution (NSS) after 30 min. n=5
5mg/kg Tacrine group: Oral administration of 3% (w/v) acacia followed by i.p. injection of 5mg/kg tacrine dissolved in NSS after 30 min. n=5
10mg/kg Sinemet treatment group:
Oral administration of 10mg/kg sinemet (Levodopa + Carbidopa in the ratio of 4:1) followed by i.p. injection of 5mg/kg tacrine dissolved in NSS after 30 min. n=5
Mice were immediately kept inside FPA for recording their behavior for 1 hr and 30 min.
Mice of MPTP group were randomly divided into:
Sham: I.p. injection of PBS was injected once a week until 5th injection. Two weeks gap was placed as watch out phase and they were again injected i.p. with PBS (6th injection). Two last injection of MPTP (7th and 8th) were injected along with oral gavage of 3% (w/v) acacia. n=5
30mg/kg MPTP group: 30mg/kg MPTP (i.p.) dissolved in PBS was injected once a week until 5th injection. Two weeks gap was placed as watch out phase and they were again injected i.p. with 30mg/kg MPTP (6th injection). Two last injection of MPTP (7th and 8th) were injected along with oral treatment of 10mg/kg Sinemet suspended in 3% (w/v) of acacia. n=5
Injections were administered once a week and mice were immediately kept inside FPA for recording for 2 hrs.
Locomotor parameters such as distance travelled and bout of low mobility (BLM) and power spectra shown by mice in order to show their Parkinsonism behavior were computed and compared with treatment group.
Statistics:
All data were expressed as mean ± standard Error (SE). Comparison between the groups was analyzed using Student’s t-test (unpaired) or ANOVA with post hoc analysis (LSD). Probability of 5% (p<0.05) was considered to be significant. All data were analyzed using IBM SPSS Statistics version 22.
RESULTS:
Locomotor activity:
Locomotion gives us an idea about the motor function of body. Under the influence of drugs like tacrine and MPTP, we hypothesized that mice should experience some motor abnormality and might not travel at all. Distance travelled and BLM was used as dependent variables to describe locomotor activity of mice. BLM is used to calculate motor activities in cases when the animal stays in one place and performs other activities like head bobbing, grooming, and rearing. BLM scale ranges from 0-8, 0 being high motor activity while 8 being the case in which the animal is not showing any kind of movement. If an animal says in a virtual circle of 15 mm radius for more than 5 sec, then BLM scale is increased.
Tacrine started to show its effect 10-15min after their i.p. administration. Locomotor activity shown by tacrine group is graphically shown in Fig. 1.
FIG. 1: DISTANCE TRAVELLED AND BLM OF MICE OF GROUP SHAM, 5mg TACRINE AND 5mg/kg TACRINE+10mg/kg SINEMET. DATA ARE PRESENTED AS THE GROUP MEANS ± SEM (n = 5 per group)
*p<0.05 as compared with Sham
**p<0.05 as compared with 5mg/kg Tacrine group
Mice injected with 5mg/kg tacrine showed significantly reduction in distance travelled (65294.24±7998.10 mm) as compared with sham (400953.3±82248.85 mm). BLM was significantly increased in case of mice injected with 5mg/kg tacrine (6.82±0.29) as compared with sham (1.096±0.33). Both of these parameters were seen to be ameliorated significantly by 10mg/kg sinemet (314558.5±102940.2 mm and 4.01±0.91) as compared with 5mg/kg tacrine group.
Like tacrine, 30mg/kg MPTP also started to show its Parkinsonism effect after 10-15 minutes of i.p. administration. Fig. 2 describes the locomotor activity of MPTP. I.p injection of 30mg/kg MPTP also reduced distance travelled significantly (80971.38±2576.56mm) as compared with sham (502886.5±86387.06mm) and increased BLM (6.88±0.16) as compared with sham (1.195±0.786).
Both of these parameters were seen to be improved by oral administration of 10mg/kg sinemet (337311.1±67183.83 mm and 4.64±0.31) as compared with 30mg/kg MPTP group.
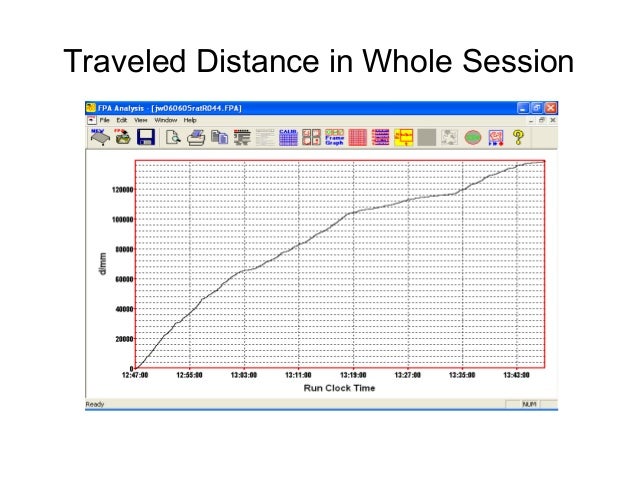
FIG.2: DISTANCE TRAVELLED AND BLM OF MICE OF GROUP SHAM, 30mg/kg MPTP (6th injection) AND 30mg/kg MPTP+10mg/kg Sinemet (8th injection). DATA ARE PRESENTED AS THE GROUP MEANS ± SEM (n = 5 per group.
*p<0.05 as compared with Sham
**p<0.05 as compared with 30mg/kg MPTP group
Power Spectra Analysis:
Time interval inside FPA was divided into different number of frames, with one frame equals to 80 sec. After each 80 sec, a new frame was started. For recording of 2hr, the time interval is divided into 88 frames. When analyzing results, we selected those frames in which the mice are not moving to reduce any kind of noise produced in power spectra; for example, paws of mice. Trajectory motion pictures show the position of mice at that frame which gives an idea about the locomotion of each mouse. Y-axis depicts the intensity of power and x-axis represents frequency in hertz (Hz). Noticeable peaks are expected to be Parkinsonism symptoms shown by mice inside FPA. Black line represents positive control (5mg/kg tacrine) and black dotted line represents 5mg/kg tacrine group
FIG.3: COMPARISON OF TRAJECTORY MOTION PICTURES AND POWER SPECTRA RESULTS OF SHAM v/s 5mg/kg TACRINE.
Trajectory motion of Sham shows the pathway of mice at the frame in which power spectra was analyzed. Because of the noise produced by paws of mice when walking around the plate, power spectra of Sham are not clear.
Power spectra of 5mg tacrine were relatively insignificant as compared with Sham group. Mouse of tacrine group was standing still as per the trajectory motion pictures.
Trajectory motion pictures and power spectra of 5mg/kg Tacrine compared with the power spectra of 10mg/kg Sinemet followed by intraperitoneal administration of 5mg/kg Tacrine:
Comparison of power spectra results of 5mg/kg tacrine with 5mg/kg tacrine+10mg/kg sinemet along with their trajectory motion pictures are shown below. Dotted line represents 5mg/kg tacrine, and black line represents 5mg/kg tacrine+10mg/kg sinemet. 5mg/kg tacrine showed significant peak at around 10-12Hz and the peak was found to be suppressed by the oral treatment of 10mg/kg sinemet. This peak was expected to be Parkinsonism symptom of tacrine (lateral movement of jaw) because it was remarkably higher than the remaining other peaks.
FIG. 4: COMPARISON OF TRAJECTORY MOTION PICTURES AND POWER SPECTRA RESULTS OF 5mg/kg tacrine v/s 5mg/kg tacrine+10mg/kg SINEMET
Trajectory motion of Sham showed the pathway of mice at the frame in which power spectra was analyzed. Because of the noise produced by paws of mice when walking around the plate, the power spectra of Sham were not clear. Power spectra of 30mg/kg MPTP are comparatively insignificant as compared with Sham group. Mouse of 30mg/kg MPTP group was standing still as per the trajectory motion pictures.
FIG. 5: COMPARISON OF TRAJECTORY MOTION PICTURES AND POWER SPECTRA RESULTS OF SHAM v/s 30mg/kg MPTP
Trajectory motion pictures and power spectra of 30mg/kg MPTP compared with the power spectra of 10mg/kg Sinemet followed by intraperitoneal administration of 30mg/kg MPTP:
Comparison of power spectra results of 30mg/kg MPTP with 30mg/kg MPTP+10mg/kg sinemet along with their trajectory motion pictures are shown below. Dotted line represents 30mg/kg MPTP and black line represents 30mg/kg MPTP +10mg/kg sinemet. 30mg/kg MPTP showed significant peak that ranged around 7-12Hz and the peak was seen to be suppressed by the oral treatment of 10mg/kg sinemet. The number of peaks was variable from 1 to 4. These peaks were expeced to be Parkinsonism symptoms of MPTP (tremor, body shaking and rigidity) because they were remarkably higher than the remaining other peaks.
FIG. 6: COMPARISON OF TRAJECTORY MOTION PICTURES AND POWER SPECTRA RESULTS OF 30mg/kg MPTP v/s 30mg/kg MPTP+10mg/kg SINEMET GROUP
DISCUSSION: Growing study of PD has suggested the role of increased acetylcholine and reduced dopamine in brain of PD patients. Lack of dopamine in nigrostriatal pathway is found to have directly linked with motor abnormalities of body 4, 5, 19. Previous studies have discovered the motor effects of cholinomimetic drugs. Drugs like pilocarpine, haloperidol 9, 12, 20 are reported to induce extra pyramidal dysfunction, catalepsy and reduction of locomotion. Another major motor effect of cholinomimetic drugs is the induction of vertical deflections of jaw which is rapid and is not stimulated by any stimulus Tacrine acts as a noncompetitive inhibitor of acetyl cholinesterase. It is thought to interact with the hydrophobic site which is adjacent to catalytic site in cholinergic system 5, 19.
Carriero et al in 1997 and Pan et al in 2006 demonstrated the dose dependent induction of tremolous jaw movement and dose dependent suppression of locomotor activity. 1.25, 2.5 and 5.0 mg/kg tacrine (i.p.) was able to induce jaw movements and suppress locomotion in rats. The number of rearing was also reduced on increasing dose 9, 21. Cousins et al in 1997 also reported that the jaw movements induced in rats are directly dependent on level of Ach in ventrolateral striatum. Administration of 2.5-5mg/kg Tacrine increased both jaw movement and extracellular level of Ach in ventro lateral striatum. As the dose of tacrine was increased, the level of Ach was also increased showing a linear correlation in the first 30 min post injection. Hence there may be a direct relationship with level of Ach in ventrolateral striatum and induction of chewing effect in rodents 12. Consistent with the previous research, i.p. injection of 5mg/kg tacrine reduced locomotion activity and increased BLM in rats in a significant amount as compared with sham. Rats also showed salivation and lateral movement of jaws. The lateral movement of jaws persisted for about one and half hour, whereas salivation was found up to 4 hrs. post injection.
Mulcahy et al in 2011 and Chia et al in 1996 found that chronic administration of MPTP induces high locomotion that is similar to sham. This may be due to the tolerance of mice against MPTP due to repeated administration. They also reported that after a single dose of MPTP, mice exhibits parkinsonism features like straub tail( bowed still tail), sialorrhoea (increased salivation and secretion from nose), hypernea (increased respiration), muscular hypotonia and teeth chatter possibly due to tremor. Consistent with previous report, MPTP treated mice of our experiment also showed similar types of response, but were usually vanished after 3-4 hours of injection.
As the dose of MPTP injected was only once a week for 8th dose, no tolerance effect was seen against MPTP because the rate of dose we injected may be too low to induce tolerance in mice. 10mg/kg sinemet (Levodopa + Carbidopa in the ratio of 4:1) was found to ameliorate the motor deficits induced by both drugs 22, 23. Interaction of dopamine and Ach in brain is an important factor for normal and coordinated movement. Disruption in any of this neurotransmitter may result in motor abnormality. Sinemet contains the precursor of dopamine, levodopa which acts by replacing the degenerated dopaminergic neurons and reestablish normal dopaminergic flow from substantia nigra to striatum. Hence, both 5mg/kg Tacrine and 30mg/kg MPTP may possibly be able to affect locomotion in C57BL/6 mice which was improved by oral administration of Sinemet.
Cousins et al in 1998 found out that the frequency of lateral movement of jaws induced by 2.5mg/kg tacrine in rats ranged from 3 to 5 Hz 12. Our result showed that the frequency of lateral movement of jaws induced by 5mg/kg tacrine in C57BL/6 mice ranged from 10-12 Hz. This variation may be due to the difference in species even though they both belong to rodent family. 30mg/kg MPTP resulted in generation of one to four peaks ranging from 8 to 12 Hz.
Comparison of power spectra of tacrine and MPTP showed that tacrine has only one narrow peak ranging from 10-12 Hz that is prominent while MPTP has one to four peaks ranging from 8 to 12 Hz. This may be because the Parkinsonism syndrome expected from 5mg/kg tacrine is lateral movement of jaw (only oral/ facial part) only whilst that of MPTP is rigidity and tremor (facial, abdominal and tail portion). Henceforth, MPTP may have much number of peaks than that of tacrine.
CONCLUSION: These findings imply that 5mg/kg tacrine and 30mg/kg MPTP can be used as a Parkinsonism model using FPA. Since tremor and motor dysfunction are classic symptoms of Parkinsonism, it is possible to use these results to create Parkinsonism model in laboratory by the use of FPA. These results may be used to test capability of new anti parkinsonian drugs to improve motor abilities in rodents.
Human: fall flat crack. ACKNOWLEDGMENT: This work was supported by the grant of Thailand Institute of Scientific and Technological Research (TISTR), Pathum Thani, Thailand.
REFERENCES:
- Rana, A.Q, Siddiqui, I; Yousuf, M.S. Challenges in diagnosis of young onset Parkinson's disease. J Neurol Sci. 2012, 323:113–116.
- Fearnley, J.M, Lees, A.J. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1991, 114:2283-301.
- Meissner, W.G, Frasier M, Gasser T, Goetz, C.G, Lozano A, Piccini P. Priorities in Parkinson’s disease research. Nat Rev.2011; 10:377–393.
- Gelb, D.J, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999 Jan; 56(1):33-9.
- Moon, J.H, Kim, J.H, Im, H.J, Lee, D.S, Park, E.J, Song, K, Oh, H.J, Hyun, S.B, Kang, S.C, Kim H, Moon, H.E, Park, H.W, Lee, H.J, Kim, E.J, Kim S, Lee, B.C, Paek, S.H. Proposed Motor Scoring System in a Porcine Model of Parkinson's Disease induced by Chronic Subcutaneous Injection of MPTP. Exp Neurobiol. 2014; 23(3):258-65. Epub 2014 Sep 18.
- Damier P, Hirsch, E.C, Agid Y, Graybiel, A.M. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain 1999; 122: 1437-1448.
- Xia R, Mao, Z.H. Progression of motor symptoms in Parkinson’s disease. Neurosci Bull. 2012, Volume 28(1):39-48.
- Pfeiffer, R.F. Non-motor symptoms in Parkinson's disease. Parkinsonism and Related Disorders (2015); doi:10.1016/j.parkreldis.2015.09.004.
- Carriero, D.L, Outslay G, Mayorga, A.J, Aberman J, Gianutsos G, Salamone, J.D. Motor dysfunction produced by tacrine administration in rats. Pharmacol Biochem Behav 1997; 58:851–8.
- Koganemaru G, Hiroshi A. Effects of cabergoline and rotigotine on tacrine-induced tremulous jaw movements in rats. Pharmacology, Biochemistry and Behavior. 2014; 126: 103–108.
- Alejandro R, Ramón C, María J.O, Abdelouahid, José MC. Novel tacrine-related drugs as potential candidates for the treatment of Alzheimer’s disease. 2013; 23 (7), 1916–1922.
- Cousins MS, Finn M, Trevitt J, Carriero DL, Conlan A, Salamone JD. The role of ventrolateral striatal acetylcholine in the production of tacrine-induced jaw movements. Pharmacol Biochem Behav. 1999 Mar;62(3):439-47
- Lyndsey ECP, Nicholas EP, Kristen LR, James RH, James JC, Patrick BS, and John DS. Pharmacological and Physiological Characterization of the Tremulous Jaw Movement Model of Parkinsonian Tremor: Potential Insights into the Pathophysiology of Tremor. Front Syst Neurosci. 2011; 5: 49.
- Prezedborski S, Jackson LV, Djaldetti R, Liberatore G, Vila M, Vukosavic S, Almer G. The parkinsonian toxin MPTP: action and mechanism. Restor Neurol Neurosci (2000) 16 135-142.
- Watanabe Y, Himeda T, Araki T. Mechanisms of MPTP toxicity and their implications for therapy of Parkinson’s Disease. Med Sci Monit 2005; 11(1): RA 17-23.
- Gregory P, Qin Li, and Erwan Bezard. Modeling Parkinson’s Disease in Primates: The MPTP Model. Cold Spring Harb Perspect Med. 2012; 2(3)
- Wang, S.C. Fowler. Concurrent quantification of tremor and depression of locomotor activitu induced rats by harmaline and physostigmine. Psychopharmacology 2001; 158:145.
- Fowler SC, Birkestrand BR, Chen R, Moss SJ, Vorontsova E, Wang G, Zarcone TJ. A force-plate actometer for quantitating rodent behaviors: illustrative data on locomotion, rotation, spatial patterning, stereotypies, and tremor. J Neurosci Methods. 2002 May 30; 107(1-2):107-24.
- Qi Sun, Da-Yong Peng, Sheng-Gang Yang, Xiao-Lei Zhu, Wen-Chao Yang, Guang-Fu Yang. Syntheses of coumarin–tacrine hybrids as dual-site acetylcholinesterase inhibitors and their activity against butylcholinesterase, Ab aggregation, and b-secretase. Bio org Med Chem. 2014 Sep 1; 22(17):4784-91.
- Miwa H. Rodent models of tremor. Cerebellum 2007; 6:66–72.
- Pan SY, Han YF, Yu ZL, Yang R, Dong H, Ko KM. Evaluation of acute tacrine treatment on passive-avoidance response, open-field behavior, and toxicity in 17- and 30-day-old mice. Pharmacology, Biochemistry and Behavior 85 (2006) 50–56
- Mulcahy P, O’Doherty A, Paucard A, O’Brien T. The behavioural and neuropathological impact of intranigral AAV-α-synuclein is exacerbated by systemic infusion of the Parkinson’s disease-associated pesticide, rotenone, in rats. Behav Brain Res.2013;15 (243):6–15
- Chia, LG, Ni, DR, Cheng, LJ, Kuo, JS, Cheng, FC, Dryhurst, G. Effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and 5,7- dihydroxytryptamine on the locomotor activity and striatal amines in C57BL/6 mice. Neurosci. Lett. 1996; 218, 67–71
How to cite this article:
Shrestha S, Phornchirasilp S and Thisayakorn K: Comparative Analysis of Drug-Induced Parkinsonism Like Behaviors In C57bl6 Black Mice Using A Force Plate Actimeter. Int J Pharm Sci Res 2016; 7(2): 873-81.doi: 10.13040/IJPSR.0975-8232.7(2).873-81.
All © 2013 are reserved by International Journal of Pharmaceutical Sciences and Research. This Journal licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License.
53
873-81
618
1042
0
English
IJPSR
Stuti Shrestha , Srichan Phornchirasilp and Krittiya Thisayakorn *
Pharmaceutical and Natural product Department , Thailand Institute of Scientific and Technological Research (TISTR), PathumThani, Thailand
annkrit@yahoo.com
01 September, 2015
18 November, 2015
Force Plate Actimeter
17 January, 2016
Actimeters Define
10.13040/IJPSR.0975-8232.7(2).873-81
Actimeter Test
01 February, 2016
